- Home >
- Applications >
- Rapid Sterility Testing
Rapid sterility testing
SHORTENING THE RELEASE TIME OF CELL AND GENE THERAPIES
- Results in less than 3 days
- Continuous phenotypic detection
- Direct inoculation of product
- Non-destructive measurement
- Small test volume requirements
Cell and gene therapies need to be available to the patient quickly, and today’s post-manufacture release times are too slow. The long turnaround times and complex procedures of traditional sterility tests lead to long vein-to-vein times, high development costs, and increased patient risks.
The Symcel calScreener+, an isothermal microcalorimeter, is designed specifically to address these issues. It provides accurate growth-based test results within three days. It requires a minimal sample volume and, since it detects only viable organisms, delivers decisive, positive/negative results. The system is highly sensitive (LOD <5 CFU), features continuous measurements, is easy to operate — and is fully compliant with industry standards.
Welcome to next-generation sterility testing.
RAPID STERILITY TESTING OF CELL AND GENE THERAPIES
Isothermal microcalorimetry detects heat with extreme sensitivity. We have optimized a microcalorimeter specifically for the easy, accurate, and fast detection of metabolically active organisms.
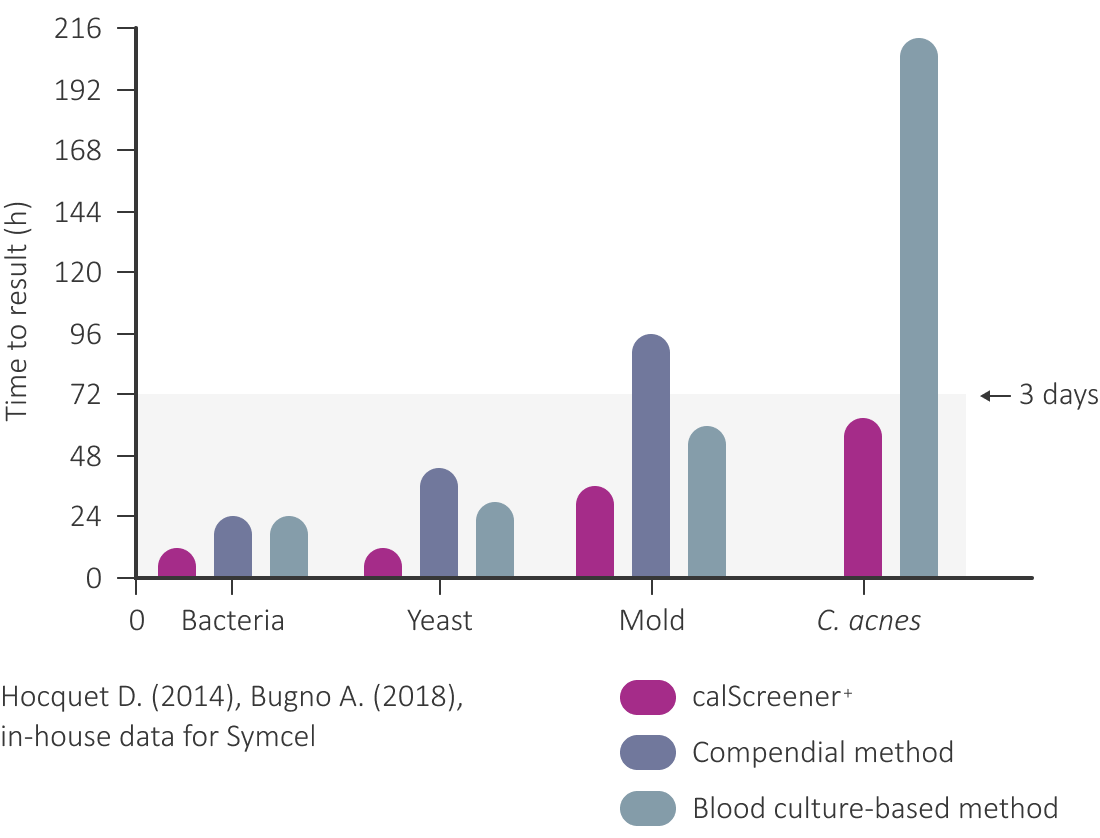
Time to detection with our biocalorimeter system is less than 60 hours in growth promotion assays of <5 CFU/mL, with C. acnes being the slowest microorganism detected at 58 hours.
This is significantly faster than compendial methods (14 days), as well as other rapid-growth microbial methods (7 days). The image to the right shows a general comparison to compendial methods and colorimetric blood culture-based methods, cutting time by around 60% while minimizing the risk of false results.
This means faster results no longer require compromising on safety or reliability, and allows you to overcome challenges associated with testing complex product matrices, simplify manufacturing processes, and slash your time to market.
rapid microbial detection
Release times are critical for life-saving therapies. Our novel biocalorimetry technology delivers faster, continuous, and more actionable results. With accurate, decisive results available in less than three days, product release decisions can be made earlier and with full confidence.
- Majority of bacteria detected in < 24 hours
- Fungi, including A.brasiliensis, in < 48 hours
- C.acnes, assay rate limiting organism, detected in < 60 hours
.jpg?width=1628&height=1120&name=Testimony%20(30).jpg)
Minimized risk of false results, even in complex backgrounds
The calScreener+ growth-based method measures continuously and since it works phenotypically, detects only viable microorganisms. It offers exceptional sensitivity, with a limit of detection (LOD) below 5 CFU, and minimizes the risk of false results caused by debris, residual genetic material, or components of the cellular product.
Where other growth-based sterility methods rely on a chain of indirect measurements, the calScreener+ offers true, more direct detection of microbial activity.

STERILITY TESTING OF COMPLEX CELL THERAPY PRODUCT MATRICES
One major challenge in testing Advanced Therapy Medicinal Products (ATMPs) is the complexity of their product matrices, which can inhibit microbial growth or mask detection signals. This often requires extensive pre-processing steps that can introduce additional variability and potentially affect the accuracy of the test results. Biocalorimetry enables direct inoculation and reliable detection of contamination—even in highly complex sample matrices. This makes the Symcel sterility test optimal for cell and gene therapy products.
- No filtering
- No sample concentration
- No extraction
.jpg?width=1200&height=800&name=Testimony%20(46).jpg)
EASIER CONTAMINATION INVESTIGATION
Root cause analysis is critical if contamination is detected. The calScreener+ method is non-destructive with no manipulation steps or interfering reagents. If a sample is found positive, inoculated samples can immediately be analyzed further using standard methods.
- No reagents
- No dyes
- No lysing
PRESERVE MORE PRODUCT FOR THE PATIENT
Traditional sterility testing methods require sample volumes that many advanced therapy products simply can’t spare. In contrast, the calScreener+ system requires only a minimal volume, enabling reliable testing even when product availability is critically low.
.jpg?width=1200&height=800&name=Testimony%20(47).jpg)
3-DAY CAR-T THERAPY STERILITY TESTING
Case study using a Jurkat CAR-T cell line. Study performed at <5 CFU in TSB and FTM growth media with direct inoculation in the presence of 106 CAR-T cells/ml. The samples were spiked with USP <71> recommended species plus C.acnes.
Data is representative of more than 10 different autologous cell therapy products tested, with fast and accurate detection, regardless of the product matrix.
.jpg?width=1628&height=1120&name=Testimony%20(27).jpg)
Strategic R&D collaboration with Johnson & Johnson
Sterility testing is the most time-consuming step in releasing cell therapies. Current methods can take 7–14 days, delaying access to critical treatments. This collaboration aims to deliver a validated, GMP-compliant test that cuts release time to under 3 days—improving manufacturing efficiency with faster access for patients.
.jpg?width=1146&height=600&name=Announcement%20(60).jpg)
CALSCREENER+ STERILITY
The calScreener+ Sterility biocalorimeter system performs tests directly in vials containing the growth medium, with no need for preparatory steps or the addition of costly reagents. The test provides continuous, phenotypic detection, delivering decisive, positive/negative results within three days.
.jpg?width=1200&height=800&name=Testimony%20(44).jpg)
VALIDATION AND REGULATORY APPROVAL
The calScreener+ Rapid Microbial Method (RMM) follow the US and EU standards USP <1223> and <1071>, PDA TR 33, EP 5.1.6 and EP 2.6.27. Primary validation package is conducted on method limit of detection (LOD), comparability, specificity, ruggedness and robustness, with data package availability before conducting method validation.
Contact us for more information and to conduct feasibility studies on your samples.






